Dementia
Adult: 1 mg tid or 4.5 mg once daily in the morning.
|
Indications and Dosage
Oral
Dementia Adult: 1 mg tid or 4.5 mg once daily in the morning.
|
|
Administration
Should be taken with food. Take just before meals.
|
|
Contraindications
Acute or chronic psychosis. Concurrent use w/ strong CYP3A4 inhibitors.
|
|
Special Precautions
Patient w/ severe bradycardia.
|
|
Adverse Reactions
Nervous: Headache, dizziness.
CV: Bradycardia, tachycardia, orthostatic hypotension. GI: Abdominal cramps, nausea, vomiting, anorexia. Resp: Nasal congestion. Ophthalmologic: Blurred vision. Dermatologic: Rash, skin flushing. |
|
Patient Counseling Information
This drug may cause dizziness, if affected, do not drive or operate machinery.
|
|
Overdosage
Symptoms: Nasal stuffiness, flushing, headache, nausea, vomiting, tremulousness, spasticity, hypotension, circulatory collapse, coma. Management: Symptomatic and supportive treatment. Empty the stomach by gastric lavage or emesis depending on the patient’s level of consciousness, and maintain adequate airway. For prevention of circulatory collapse, appropriate positioning of patient, fluids and, if necessary, vasopressor agents may be beneficial.
|
|
Drug Interactions
May reduce plasma concentration of ticlopidine. Enhanced serotonergic effect w/ MAOIs, linezolid. May enhance the adverse effect of antipsychotic agents, metoclopramide. Enhanced vasoconstriction w/ beta-blockers. May diminish the vasodilatory effect of nitroglycerin.
Potentially Fatal: Increased plasma concentration, leading to ergotism, when used w/ potent CYP3A4 inhibitors including protease inhibitors (e.g. boceprevir, darunavir, ritonavir), azole antifungals (e.g. ketoconazole, itraconazole, voriconazole), and some macrolide antibiotics (e.g. erythromycin, clarithromycin, telithromycin). |
|
Action
Description:
Mechanism of Action: Co-dergocrine mesylate is a mixture of hydrogenated derivatives of ergot alkaloids, including dihydroergocornine, dihydroergocristine, and dihydroergocryptine (dihydro-α-ergocryptine and dihydro-β-ergocryptine in 2:1 ratio). It was originally classed as a peripheral and cerebral vasodilator, but now considered as a metabolic enhancer. However, the exact mechanism of action by which it produces mental effects has not been determined. Pharmacokinetics: Absorption: Rapidly absorbed from the GI tract. Bioavailability: 25%. Time to peak plasma concentration: 1.5-3 hr. Distribution: Plasma protein binding: 81%. Metabolism: Undergoes extensive hepatic first-pass metabolism. Excretion: Mainly via faeces and also in urine (approx 2 %). Elimination half-life: Biphasic: 1.5-2.5 hr (α phase); 13-15 hr (β phase). |
|
Chemical Structure
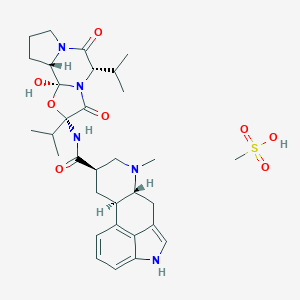 Source: National Center for Biotechnology Information. PubChem Database. Hydergine, CID=168870, https://pubchem.ncbi.nlm.nih.gov/compound/Hydergine (accessed on Jan. 21, 2020) |
|
Storage
Store between 20-25°C.
|
|
References
Anon. Ergoloid Mesylates . Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 04/04/2017. Buckingham R (ed). Codergocrine Mesilate. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 04/04/2017. Ergoloid Mesylates Tablet (Mutual Pharmaceutical Company, Inc). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 04/04/2017. McEvoy GK, Snow EK, Miller J et al (eds). Ergoloid Mesylates. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 04/04/2017. Preston CL (ed). Codergocrine Interactions. Stockley’s Drug Interactions [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 18/04/2017.
|